A survey of the role of nitrile groups in protein-ligand interactions.
1School of Pharmaceutical Science & Technology, Tianjin University, Tianjin 300072, PR China.
2National Institute of Biological Sciences, Beijing, No. 7 Science Park Road, Zhongguancun Life Science Park, Beijing 102206, PR China.
3Tsinghua Institute of Multidisciplinary Biomedical Research, Tsinghua University, Beijing 102206, PR China
Abstract
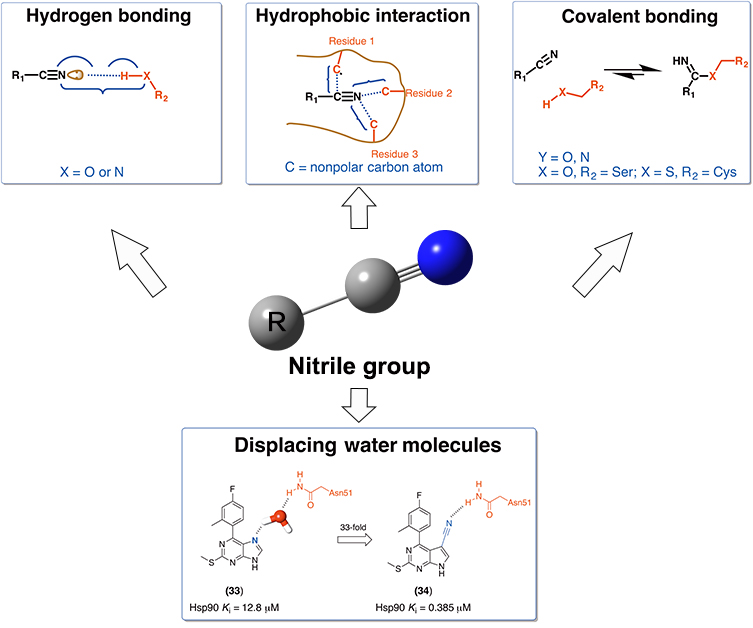
In classical medicinal chemistry, nitrile groups were commonly considered as bioisosteres of carbonyl, hydroxyl and carboxyl groups, as well as halogen atoms. However, there is a lack of in-depth understanding about the structural and energetic characteristics of nitrile groups in protein-ligand interactions. Here, we have surveyed the Protein Data Bank and ChEMBL databases with the goal of characterizing such protein-ligand interactions for nitrile-containing compounds. We discuss the versatile roles of nitrile groups in improving binding affinities, and give special attention to examples of displacing and mimicking binding-site waters by nitrile groups. We expect that this review article will further inspire medicinal chemists to exploit nitrile groups rationally in structure-based drug design.
