
NIBS黄牛实验室、张二荃实验室和BIG杨运桂实验室合作发现FTO抑制剂entacapone通过调控FTO-FOXO1通路治疗代谢综合征

Posted by Luyao Ma on April 18, 2019
Posted in news

NIBS黄牛实验室、张二荃实验室和BIG杨运桂实验室合作发现FTO抑制剂entacapone通过调控FTO-FOXO1通路治疗代谢综合征
Posted by admin on August 23, 2017
Posted in news
Posted by admin on June 16, 2017
Posted in news
| Title | Author | Cited |
|---|---|---|
|
RA Friesner, RB Murphy, MP Repasky… – Journal of medicinal …, 2006 |
1728 | |
|
GL Warren, CW Andrews, AM Capelli… – Journal of medicinal …, 2006 |
1230 | |
|
N Huang, BK Shoichet, JJ Irwin – Journal of medicinal chemistry, 2006 |
920 | |
|
W Sherman, T Day, MP Jacobson… – Journal of medicinal …, 2006 |
897 | |
|
J Wang, SM Soisson, K Young, W Shoop, S Kodali… – Nature, 2006 |
680 | |
|
K Ding, Y Lu, Z Nikolovska-Coleska… – Journal of medicinal …, 2006 |
512 | |
|
M Matsumoto, H Hashizume, T Tomishige… – PLoS Med, 2006 |
|
491 |
|
HA Overton, AJ Babbs, SM Doel, MCT Fyfe… – Cell …, 2006 |
|
476 |
|
MS Karthikeyan, DJ Prasad, B Poojary… – … & medicinal chemistry, 2006 |
436 | |
|
M Whiting, J Muldoon, YC Lin… – Angewandte Chemie …, 2006 |
427 |
Posted by admin on December 12, 2016
Posted in news

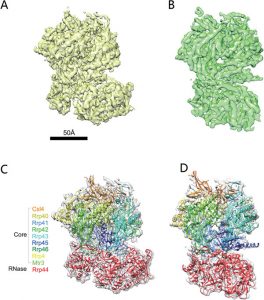 CryoEM structure of yeast cytoplasmic exosome complex.
CryoEM structure of yeast cytoplasmic exosome complex.
Jun-Jie Liu1,2,*, Chu-Ya Niu1,*, Yao Wu3,*, Dan Tan3, Yang Wang1, Ming-Da Ye1, Yang Liu2,4, Wenwei Zhao4, Ke Zhou5, Quan-Sheng Liu5, Junbiao Dai6, Xuerui Yang4, Meng-Qiu Dong3, Niu Huang3 and Hong-Wei Wang1
Posted by admin on January 6, 2016
Posted in news
 Discovery of 2-Acylaminothiophene-3-Carboxamides as Multitarget Inhibitors for BCR-ABL Kinase and Microtubules.
Cao R1, Wang Y1, Huang N1.
1National Institute of Biological Sciences, Beijing , No. 7 Science Park Road, Zhongguancun Life Science Park, Beijing, 102206, China.
Abstract
The emergence of drug resistance of the BCR-ABL kinase inhibitor imatinib, especially toward the T315I gatekeeper mutation, poses a great challenge to targeted therapy in treating chronic myeloid leukemia (CML) patients. To discover novel inhibitors against drug-resistant CML bearing T315I mutation, we applied a physics-based hierarchical virtual screening approach to dock a large chemical library against ATP binding pockets of both wild-type (WT) and T315I mutant ABL kinases in a combinatorial fashion. This strategy automatically resulted in 87 compounds satisfying structural and energetic criteria of both WT and T315I mutant kinases. Among them, nine compounds, which share a common thiophene-based scaffold and adopt similar binding poses, were chosen for experimental testing and one of them was shown to have low micromolar inhibition activities against both WT and mutant ABL kinases. Structure-activity relationship analysis with a series of structural modifications based on 2-acylaminothiophene-3-carboxamide scaffold supports our predicted binding mode. Interestingly, the same chemical scaffold was also enriched in our previous virtual screening campaign against colchicine site of microtubules using the same computational protocol, which suggests our virtual screening strategy is capable of discovering small-molecule ligands targeting distinct protein binding sites without sharing any sequential and structural similarity. Furthermore, the multitarget inhibition activity of this class of compounds was assessed in cellular experiments. We expect that the 2-acylaminothiophene-3-carboxamide scaffold may serve as a promising starting point for developing multitarget inhibitors in cancer treatment by targeting both kinases and microtubules.
Discovery of 2-Acylaminothiophene-3-Carboxamides as Multitarget Inhibitors for BCR-ABL Kinase and Microtubules.
Cao R1, Wang Y1, Huang N1.
1National Institute of Biological Sciences, Beijing , No. 7 Science Park Road, Zhongguancun Life Science Park, Beijing, 102206, China.
Abstract
The emergence of drug resistance of the BCR-ABL kinase inhibitor imatinib, especially toward the T315I gatekeeper mutation, poses a great challenge to targeted therapy in treating chronic myeloid leukemia (CML) patients. To discover novel inhibitors against drug-resistant CML bearing T315I mutation, we applied a physics-based hierarchical virtual screening approach to dock a large chemical library against ATP binding pockets of both wild-type (WT) and T315I mutant ABL kinases in a combinatorial fashion. This strategy automatically resulted in 87 compounds satisfying structural and energetic criteria of both WT and T315I mutant kinases. Among them, nine compounds, which share a common thiophene-based scaffold and adopt similar binding poses, were chosen for experimental testing and one of them was shown to have low micromolar inhibition activities against both WT and mutant ABL kinases. Structure-activity relationship analysis with a series of structural modifications based on 2-acylaminothiophene-3-carboxamide scaffold supports our predicted binding mode. Interestingly, the same chemical scaffold was also enriched in our previous virtual screening campaign against colchicine site of microtubules using the same computational protocol, which suggests our virtual screening strategy is capable of discovering small-molecule ligands targeting distinct protein binding sites without sharing any sequential and structural similarity. Furthermore, the multitarget inhibition activity of this class of compounds was assessed in cellular experiments. We expect that the 2-acylaminothiophene-3-carboxamide scaffold may serve as a promising starting point for developing multitarget inhibitors in cancer treatment by targeting both kinases and microtubules.